Magnesium oxide (MgO) has a high melting point because of its strong ionic bonding and the arrangement of its ions in a crystal lattice structure.
To understand this, you need to consider the nature of the chemical bonds in magnesium oxide.
Ionic Bonding: Magnesium oxide is composed of magnesium ions (Mg²⁺) and oxide ions (O²⁻). These ions have opposite charges, and they are held together by strong electrostatic attractions due to their opposite charges.
The ionic bond between them is strong, and it requires a significant amount of energy to break these attractions.
Crystal Lattice Structure: In a solid state, magnesium oxide forms a crystal lattice structure, where the positively charged magnesium ions are surrounded by negatively charged oxide ions, and vice versa.
This arrangement maximizes the attraction between the oppositely charged ions.
To melt a substance, you need to supply enough thermal energy to overcome the forces holding the particles (in this case, ions) together.
In the case of magnesium oxide, the ionic bonds are very strong, and the crystal lattice structure is stable, which means it takes a substantial amount of energy to disrupt these forces and allow the substance to change from a solid to a liquid.
That’s why magnesium oxide has a high melting point, around 2,852 degrees Celsius (5,166 degrees Fahrenheit).
The Nature of Ionic Bonding
Explanation of Ionic Bonding:
Ionic bonding happens when two atoms or ions with very different electronegativities
bond. Electronegativity measures how well an atom attracts and holds electrons.
In an ionic bond, a metal atom usually loses electrons, becoming a positively charged ion (cation), while a non-metal atom gains these electrons, becoming a negatively charged ion (anion).
The swapping of electrons causes a pull between the oppositely charged ions, leading to the creation of an ionic compound.
The electrostatic force of attraction between the cations and anions holds the ionic compound together, and this force is what gives rise to the characteristic properties of ionic compounds, such as high melting and boiling points, electrical conductivity in the molten or dissolved state, and the formation of crystalline structures.
How it Applies to Magnesium Oxide:
Magnesium oxide (MgO) is a classic example of an ionic compound. It is composed of magnesium cations (Mg²⁺) and oxide anions (O²⁻).
In the formation of magnesium oxide, magnesium atoms (a metal) lose two electrons to become Mg²⁺ ions, while oxygen atoms (a non-metal) gain two electrons to become O²⁻ ions.
The electrostatic attraction between these differently charged ions keeps them connected in a closely packed, three-dimensional lattice structure.
The chemical formula for making magnesium oxide is: 2Mg + O2 → 2MgO
The strong ionic bonds in magnesium oxide result in several key properties:
Magnesium oxide has an exceptionally high melting point (approximately 2,800°C) because the strong ionic bonds it forms need a lot of energy to break.
Electrical conductivity: In its solid state, magnesium oxide does not conduct electricity because the ions are locked in place.
However, when molten or dissolved in water, it can conduct electricity because the ions become mobile and can carry an electric current.
Hardness and brittleness: Ionic compounds like magnesium oxide are often hard and brittle due to the strong electrostatic forces between ions. When a force is applied to them, the ions shift, causing the lattice to fracture.
Importance of Strong Ionic Bonds: Strong ionic bonds are important for various reasons:
- Stability: The strong electrostatic forces between ions in an ionic compound make the compound stable and resistant to chemical decomposition under normal conditions.
- High melting and boiling points:
- Ionic compounds, with their elevated melting and boiling points, find utility in high-temperature settings, such as in refractory materials or as protective coatings.
- Electrical conductivity: Ionic compounds can conduct electricity when molten or dissolved, which is essential in applications like electrolysis, batteries, and electrochemical processes.
- Crystalline structures: The strong ionic bonds result in the formation of well-defined, repeating crystal structures, which contribute to the characteristic appearance and properties of ionic compounds.
In summary,
Magnesium oxide is a prime example of an ionic compound with strong ionic bonds, leading to its unique properties.
Strong ionic bonds are essential in various industrial and chemical applications due to their stability and distinctive characteristics.
Crystal Lattice Structure
Description of the Crystal Lattice Structure in MgO:
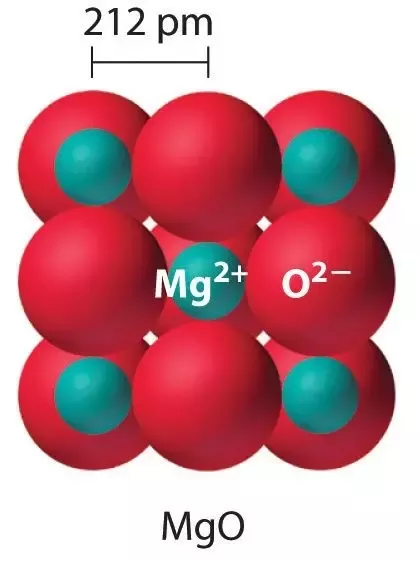
The crystal lattice structure of magnesium oxide (MgO) is a three-dimensional arrangement of ions held together by strong ionic bonds. In MgO, magnesium ions (Mg²⁺) and oxide ions (O²⁻) are arranged in a regular, repeating pattern.
The crystal lattice structure of MgO can be described as follows:
- Close-Packed Arrangement: The ions are arranged in a closely packed manner, similar to the structure of sodium chloride (table salt).
This arrangement maximizes the electrostatic attraction between the oppositely charged ions.
- Alternating Layers: The magnesium ions and oxide ions are arranged in alternating layers, with magnesium ions surrounded by six oxide ions and vice versa.
- Coordination Number: The coordination number for magnesium ions and oxide ions is 6. This implies that each ion is surrounded by six ions of the opposite charge, forming a regular octahedral arrangement.
- Repeating Unit Cell: The crystal structure repeats in three dimensions, forming a repeating unit cell.
The repeating unit cell represents the smallest portion of the crystal lattice that, when repeated in all directions, generates the entire crystal structure.
How the Structure Contributes to the High Melting Point:
The crystal lattice structure of magnesium oxide plays a crucial role in contributing to its exceptionally high melting point. Several factors can explain how the structure contributes to this property:
Strong Ionic Bonds: The high melting point of MgO is primarily due to the strong ionic bonds between the magnesium and oxide ions. These bonds are formed by the electrostatic attraction between the oppositely charged ions.
The ions in the crystal lattice are held in fixed positions, and a considerable amount of energy is required to break these bonds and convert the solid into a liquid. This energy is what we perceive as the melting point.
Three-Dimensional Lattice: The three-dimensional, closely packed lattice structure ensures that every ion is surrounded by multiple oppositely charged ions, maximizing the electrostatic forces holding them together.
This tight packing makes it more difficult for the ions to move relative to each other, further contributing to the high melting point.
Repetitive Structure: The repeating pattern of the crystal lattice extends throughout the entire solid. This means that breaking the crystal lattice requires disrupting this repetitive arrangement of ions.
The consistency and regularity of the lattice make it difficult to break apart and contributes to the high stability of the solid.
High Coordination Number: The fact that each magnesium ion is surrounded by six oxide ions and vice versa means that each ion is strongly attracted to multiple ions of the opposite charge.
This high coordination number results in a high level of structural stability.
In summary, the crystal lattice structure of magnesium oxide, with its close-packed arrangement, alternating layers of ions, high coordination number, and strong ionic bonds, is responsible for its high melting point.
The strong electrostatic forces between ions in the lattice require a significant input of energy to overcome, making magnesium oxide a high-melting-point compound with a stable, crystalline structure.
Factors Influencing Melting Points
Comparison with Other Materials:
The melting points of materials can vary significantly due to a variety of factors. Here, I will compare magnesium oxide (MgO) with other materials to illustrate the influence of different factors on melting points.
Ionic Compounds:
As we’ve talked about, magnesium oxide is an ionic compound with a high melting point, around 2,800°C.
Other ionic compounds, like sodium chloride (table salt) and calcium oxide (quicklime), similarly have high melting points because of the robust ionic bonds maintaining their crystal lattice structures.
Covalent Compounds: Covalent compounds, like molecular substances (e.g., water, methane), typically have lower melting points compared to ionic compounds.
This is because they are held together by covalent bonds, which are generally weaker than ionic bonds. For example, water (H2O) melts at 0°C, and methane (CH4) melts at around -182°C.
Metallic Compounds: Metals have relatively high melting points as well. The strength of metallic bonds, which involve the sharing of electrons in a sea of delocalized electrons, contributes to their high melting points.
For instance, iron (Fe) has a melting point of around 1,535°C, while tungsten (W) has a melting point of about 3,422°C.
Network Covalent Solids: Some covalent solids, like diamond (carbon) and silicon dioxide (quartz), form a network covalent structure in which each atom is covalently bonded to multiple neighboring atoms.
These materials have extremely high melting points due to the strong covalent bonds. Diamond has a melting point of around 3,550°C, and quartz can withstand temperatures up to around 1,700°C.
The Role of Bond Strength in Determining Melting Points:
Bond strength is a crucial factor in determining the melting points of substances.
The strength of chemical bonds, whether they are ionic, covalent, or metallic, directly influences the energy required to overcome these bonds and transition a substance from a solid to a liquid. Here’s how bond strength affects melting points:
Ionic Bonds: Substances with strong ionic bonds, like MgO, have high melting points because a significant amount of energy is needed to break the electrostatic attraction between the oppositely charged ions.
The stronger the ionic bonds, the higher the melting point.
Covalent Bonds: Substances with strong covalent bonds, especially in network covalent solids like diamond and quartz, have extremely high melting points. This is because breaking covalent bonds requires a substantial amount of energy.
Metallic Bonds: Metals have relatively high melting points due to the strength of metallic bonds, which involve a sea of delocalized electrons that hold metal ions together.
The strength of these bonds contributes to the stability and high melting points of metals.
In summary, the type and strength of chemical bonds within a substance play a significant role in determining its melting point.
Substances with strong bonds, whether they are ionic, covalent, or metallic, generally have higher melting points, whereas substances with weaker bonds tend to have lower melting points.
Comparing different materials helps illustrate how various factors, including bond strength, influence melting points.
Real-world Applications
Where Magnesium Oxide’s High Melting Point is Utilized:
Refractory Materials: Magnesium oxide is extensively employed in making refractory materials—those capable of enduring extremely high temperatures without significant deformation or deterioration.
The high melting point of magnesium oxide makes it an excellent choice for manufacturing refractory bricks, linings for furnaces, and crucibles used in industries such as steel production, glass manufacturing, and cement production.
These materials are essential for maintaining the structural integrity of equipment and containment vessels operating at extremely high temperatures.
Electrical Insulators: The high melting point and electrical insulating properties of magnesium oxide are valuable in electrical and electronic applications.
It is used as an insulating material in high-temperature electrical cables, particularly in the wiring of electric heaters and industrial ovens. The high-temperature stability of magnesium oxide ensures the safe operation of these systems.
Catalysis: Magnesium oxide is employed as a catalyst support in various chemical processes, including hydrocarbon cracking, ammonia production, and oxidation reactions.
Its high melting point is an advantage in processes involving elevated temperatures, where other materials might degrade or melt.
Medical and Dental Applications: In the medical and dental fields, magnesium oxide is used in the manufacture of dental cements, dental fillings, and prosthetic devices.
Its high melting point and biocompatibility make it suitable for dental and medical applications, where it can withstand the elevated temperatures encountered during dental procedures and sterilization.
Importance in High-Temperature Industries:
Magnesium oxide’s high melting point is of significant importance in industries that operate at elevated temperatures. Some key high-temperature industries where magnesium oxide plays a crucial role include:
Steel Manufacturing: The steel industry relies on refractory materials made from magnesium oxide to line the furnaces used for melting and shaping steel.
These refractories can withstand the extreme temperatures required for steel production.
Glass Manufacturing: Glass production involves the heating and melting of raw materials at high temperatures.
Magnesium oxide refractories are used to line glass furnaces, ensuring the containment of molten glass and preventing contamination of the glass products.
Cement Production: Cement kilns operate at high temperatures to produce clinker, a key component of cement.
Magnesium oxide-based refractory materials are utilized to line the kilns and maintain their integrity under intense heat.
Petrochemical and Chemical Industries: Many chemical processes require high-temperature conditions for reactions to occur.
Magnesium oxide catalyst supports and refractories are employed in these industries to facilitate reactions and maintain structural integrity at elevated temperatures.
Semiconductor Manufacturing: High-temperature processes are used in semiconductor fabrication.
Magnesium oxide insulators are utilized to ensure the safe operation of equipment in clean room environments where semiconductor devices are produced.
Energy Generation: Industries related to power generation, including coal-fired power plants and certain types of waste incineration, often use magnesium oxide-based materials in combustion chambers and exhaust systems due to their ability to withstand high temperatures.
In summary, magnesium oxide’s high melting point is essential in various high-temperature industries, ensuring the structural integrity and performance of equipment and materials that operate under extreme heat conditions.
Its applications range from refractory linings to electrical insulation and catalysis in industries such as steel production, glass manufacturing, petrochemicals, and more.
FAQs
What is the melting point of magnesium oxide?
Magnesium oxide has a melting point of approximately 2,852 degrees Celsius (5,166 degrees Fahrenheit).
What type of chemical bonding is responsible for the high melting point of magnesium oxide?
The high melting point is a result of strong ionic bonding between magnesium and oxygen ions.
How do ionic bonds contribute to the high melting point of magnesium oxide?
Ionic bonds involve strong electrostatic attractions between oppositely charged ions, requiring a substantial amount of energy to break these attractions, resulting in a high melting point.
What is the crystal lattice structure in magnesium oxide, and how does it affect the melting point?
Magnesium oxide forms a tightly packed crystal lattice structure, maximizing the attraction between ions, which makes it more difficult to break the structure, hence the high melting point.
Is the high melting point of magnesium oxide common among ionic compounds?
Yes, many ionic compounds have high melting points due to the strong ionic bonds and crystal lattice structures.
How does the melting point of magnesium oxide compare to other common substances?
Magnesium oxide’s melting point is significantly higher than that of most common substances, including metals, due to its strong ionic bonding.
What practical applications benefit from magnesium oxide’s high melting point?
Magnesium oxide is used in applications where high-temperature resistance is required, such as in furnace linings, kilns, and refractory materials.
Can the melting point of magnesium oxide be lowered by altering its structure or composition?
The melting point is primarily determined by the nature of ionic bonding, so significant changes to the structure or composition are unlikely to substantially lower it.
What are the consequences of magnesium oxide’s high melting point for industrial processes?
The high melting point makes magnesium oxide a valuable material for industries that involve extreme heat, as it can withstand and retain its properties at high temperatures.
Are there any downsides to magnesium oxide’s high melting point in certain applications?
In some cases, the high melting point can make it challenging to process or work with magnesium oxide, as it requires specialized equipment and techniques for melting and shaping.
Conclusion
In conclusion, magnesium oxide (MgO) is a versatile compound with a high melting point that finds widespread use in numerous real-world applications, particularly in high-temperature industries.
The strong ionic bonds and high melting point of Magnesium oxide makes it essential in various fields, including refractory materials, electrical insulation, and catalysis, as well as in industries like steel, glass, cement, petrochemicals, semiconductors, and energy generation, where it helps equipment endure extreme temperatures and perform well.
Magnesium oxide’s contribution to these applications highlights its vital role in facilitating technological advancements and maintaining the reliability of industrial operations in the face of high-temperature challenges.